
| Size (♯) | 00 | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Volume (mL) | 0.93 | 0.68 | 0.49 | 0.37 | 0.28 | 0.21 |
Pharmaceutical capsules

A plant-derived hypromellose capsule designed for use with dry powder inhalers.
Available in 6 sizes, from 00 to 4.
In addition, please contact us for inquiries about the special size.

| Size (♯) | 00 | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Volume (mL) | 0.93 | 0.68 | 0.49 | 0.37 | 0.28 | 0.21 |
The standard numbers of capsules packed in each case for outside of Japan are as follows.
| Capsule size (♯) | Standard quantities |
|---|---|
| 00 | 70,000 |
| 0 | 90,000 |
| 1 | 100,000 |
| 2 | 150,000 |
| 3 | 200,000 |
| 4 | 250,000 |
While gelatin capsules leave powder residue even at high inhalation flow rates, QUALI-V®-I capsules minimize powder residue at all flow rates, making
them ideal for inhalational APIs.
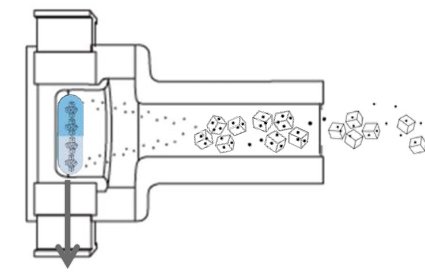
| Flow rate | Gelatin Capsules |
HPMC Capsules |
QUALI-V®-I |
|---|---|---|---|
| 30 L/min | 1.9±0.4 | 1.1±0.2 | <LOQ |
| 60 L/min | 2.1±0.2 | 1.1±0.3 | <LOQ |
| 100 L/min | 2.3±0.5 | <LOQ | <LOQ |
Inhaler: Axahaler®
Inhalation powder: 0.05% formoterol mixture
Fill weight: 24±1 mg
We prepare accreditations and/or certifications for QUALI-V®-I. For further reference, please contact us for further information.
| Kosher Certification (Copy) | ||
| Residual Solvents Statement | BSE/TSE Statement | Elemental Impurities Statement |
| GMO Statement | Allergen Statement | Melamine Statement |
| Nitrosamines Statement | Titanium Oxide Statement | Gluten Statement |
| Irradiation Statement | ||
| SDS | Capsule Specifications | Flow of Manufacturing Capsules |
| Test Methods | Capsule Theoretical Formula | COA Sample |
Additionally, Qualicaps® received ISO9001 and ISO14001 certification.
See ″ISO Management″ for further details.
QUALI-V is a registered trademark of Qualicaps in China, India, US, Canada, EU, Switzerland, and UK.