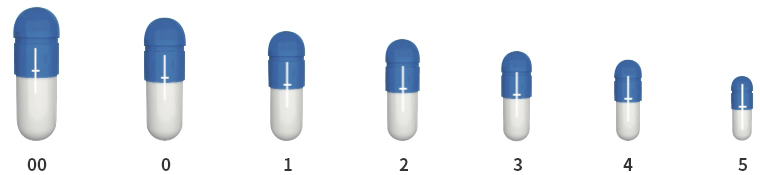
| Size (♯) | 00 | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| Volume (mL) | 0.93 | 0.68 | 0.49 | 0.37 | 0.28 | 0.21 | 0.13 |
Pharmaceutical capsules

The first hypromellose (HPMC) -based capsules, developed by Qualicaps®.
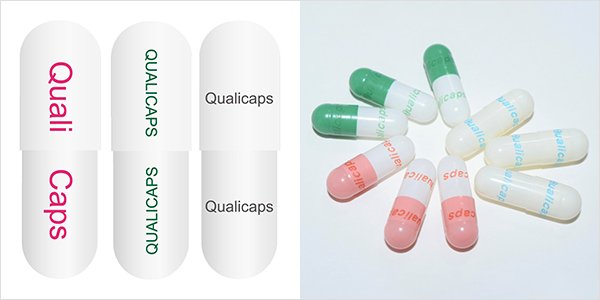
More than 90% pharmaceutical capsules made in Qualicaps® are imprinted to enhance distinguishability.
Two imprinting methods are available:
・Offset printing with various color ink
・UV lazer printing with no ink : Qualicaps®'s own technology
Offset printing uses edible ink,
in which various colors are available.
UV lazer printing technology utilizes the color change of titanium oxide by UV irradiation.
UV lazer can print fine design to capsules.
| Offset printing | UV lazer printing | |
|---|---|---|
| Color | Various | Gray |
| Fineness | Good | Excellent |
Available in 7 sizes, from 00 to 5.
In addition, please contact us for inquiries about the special size.

| Size (♯) | 00 | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| Volume (mL) | 0.93 | 0.68 | 0.49 | 0.37 | 0.28 | 0.21 | 0.13 |
Also available in size 9 for small animal use.

| Capsules for small animal use. | |
|---|---|
| Capsule sizes (♯) | 9 |
| Capacity | 25 μL |
| Colors | Cap: Clear blue Body: Clear |
The standard numbers of capsules packed in each case for outside of Japan are as follows.
| Capsule size (♯) | Standard quantities | |
|---|---|---|
| 00 | 70,000 | |
| 0 | 90,000 | |
| 1 | Transparent capsules | 100,000 |
| Opaque capsules | 125,000 | |
| 2 | 150,000 | |
| 3 | 200,000 | |
| 4 | 250,000 | |
| 5 | 400,000 | |
No difference in the rate of dissolution of QUALI-V® in water, 1st fluid for dissolution test (simulated gastric fluid), or 2nd fluid for dissolution test
(simulated intestinal fluid).
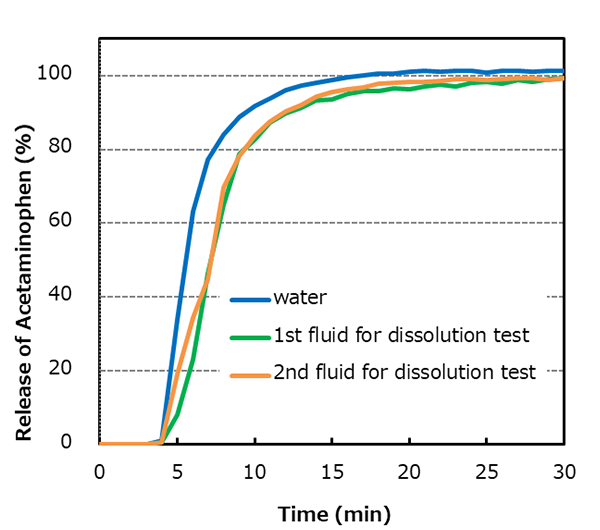
Capsule size: ♯3
Apparatus: Paddle method, 50 rpm, UV detection
Dissolution media: water, 1st fluid for dissolution test, or 2nd fluid for dissolution test, 900 mL each
Capsule fill formulation: 20% acetaminophen mixture
Fill weight: 150 mg
Capsules are evaluated by pressing 100 capsules at 0.37 MPa for 15 seconds in a dedicated testing apparatus and checking for broken capsules.
QUALI-V® capsules are more resistant to brittleness than QUALI-G™ capsules when compared at the same moisture content, which is an advantage when handling hygroscopic APIs.
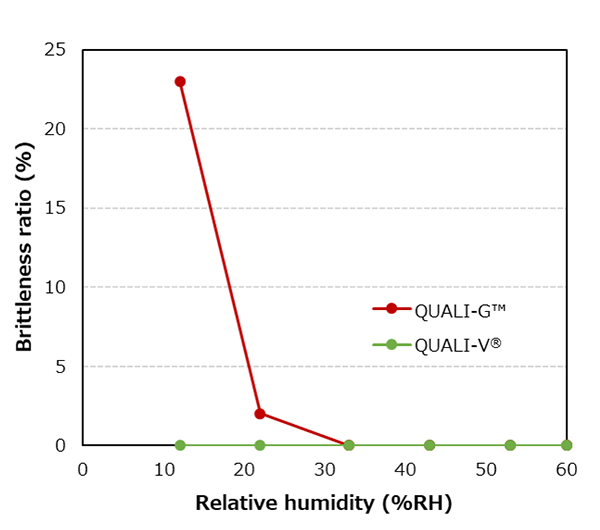
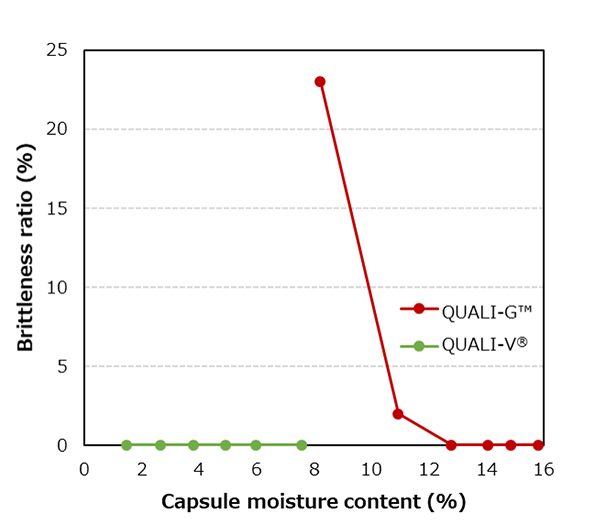
Temperature: 25.0±3.0 ℃
We prepare accreditations and/or certifications for QUALI-V®. For further reference, please contact us for further information.
| Kosher Certification (Copy) | ||
| Residual Solvents Statement | BSE/TSE Statement | Elemental Impurities Statement |
| GMO Statement | Allergen Statement | Melamine Statement |
| Nitrosamines Statement | Titanium Oxide Statement | Gluten Statement |
| Irradiation Statement | ||
| SDS | Capsule Specifications | Flow of Manufacturing Capsules |
| Test Methods | Capsule Theoretical Formula | COA Sample |
Additionally, Qualicaps® received ISO9001 and ISO14001 certification.
See ″ISO Management″ for further details.
QUALI-V is a registered trademark of Qualicaps in China, India, US, Canada, EU, Switzerland, and UK.