Capsules and Printing Ink

Quality Initiatives
We are constantly striving to improve the quality of our products and services to accurately meet customer needs with the aim of contributing to improvement of people's health.
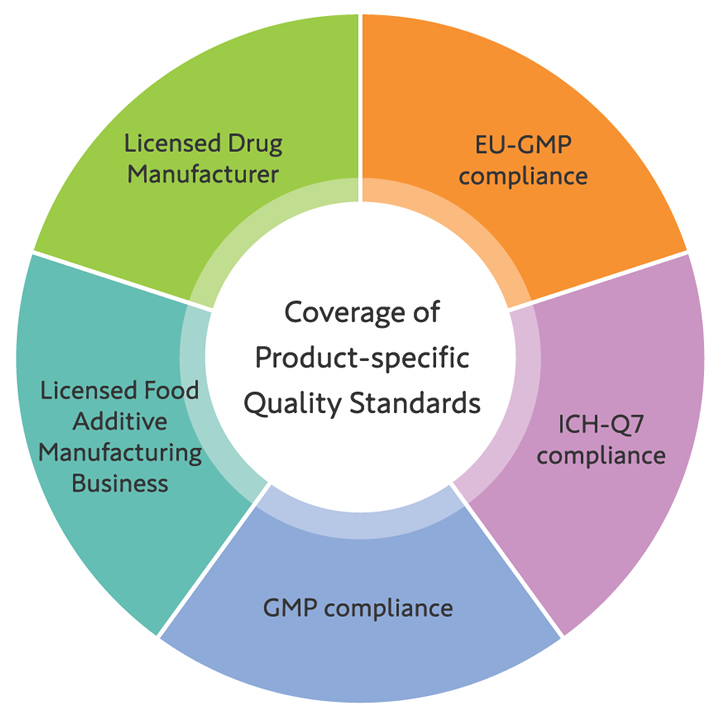
Compliance with Regulations
We manufacture and sell our products in accordance with the respective quality standards, as we manufacture capsules for pharmaceutical and health food use.

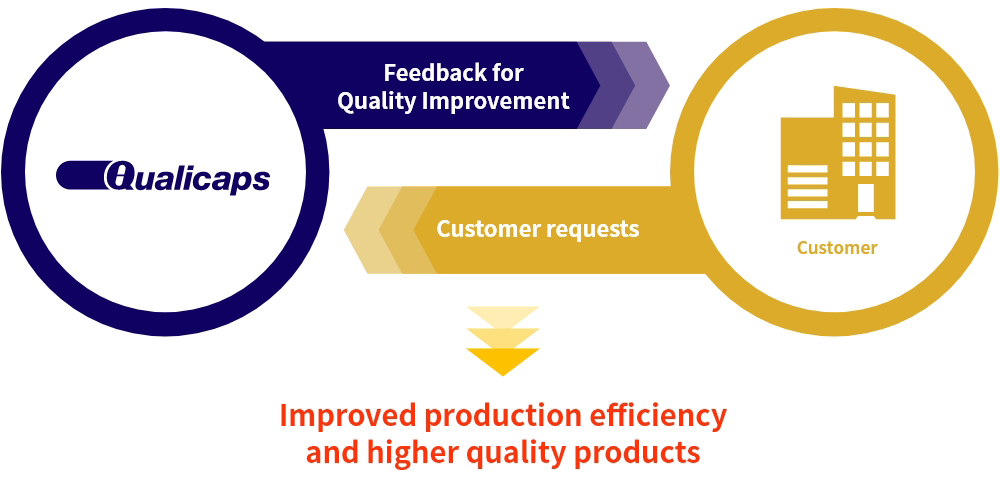
Follow-up Service
Qualicaps not only manufactures products according to customers' requests, but also welcomes feedback to further improve quality and develop better products.
Quality Control and Quality Assurance
From receiving raw materials through production, process control, and product shipment, we have established a management system to ensure that our products can be used with confidence.
Here is an example of the flow from manufacturing to shipment of capsules.
*Click on “Read More” to see further detail on quality control and quality assurance.
*Click on the “Down” icon to view detailed information on quality control and quality assurance.
Receipt of raw materials
Quality Assurance
We select and evaluate suppliers, conduct regular audits, evaluate raw material prototypes, manage changes that may affect quality, sign onto quality standards, and research any raw materials used.
Raw Material Acceptance Test
Quality Control
Acceptance tests are conducted to ensure that each raw material complies with the established specifications.
-

Chemical tests (moisture content) -

Microbial limit test (specified microorganisms)
Manufacturing of capsules
Quality Assurance
Quality assurance includes checking of manufacturing and testing records in manufacturing and quality control, and control of OOS, OOT, and deviations.
First 100% Visual Inspection / In-process Tests
Quality Control
Not only the final products, but also intermediates within the capsule manufacturing process are sampled daily and tested for moisture content, mass, and microbial limits.
We thus strive to ensure uniform quality during the manufacturing process.
-

Physicochemical test (Loss on drying) -
Microbial limit test (total aerobic microbial count)
Capsule Printing
Second 100% Visual Inspection
Packaging
Final Product Testing
Quality Control
We focus not only on GMP equipment and facilities, but also on the intangible, conducting appearance tests, physical and chemical tests, and microbial limit tests for final product testing, making daily efforts to deliver safe, reliable, and high-quality products.
-

Visual appearance test -

Physicochemical test (Dimensional test) -

Physicochemical test (solubility test) -
Microbial limit test (total aerobic microbial count)
Shipment
Quality Assurance
Release assessment is made by reviewing manufacturing records, test records, packaging records, test results, etc.
We also work constantly to improve the quality of our products in accordance with our obligation to communicate with customers through change and deviation management, and quality information processing (complaint) management for shipped products.