Capsules FAQ
Capsule Basic FAQ
- What are the difference between hard capsules and soft capsules?
- Hard capsules comprise of two pieces—a cap and a body—and are filled with a range of substances from powders to liquids. In contrast, soft capsules are a single piece that are made with a film wrapping the liquid or solid content.
- Pharmaceuticals and health foods are available as tablets and many other shapes, but what are advantages of hard capsules?
- Hard capsules have many advantages, including shortened development time, protection of filling and enhanced distinguishability.
Please see “Advantages of Hard Capsules” for details. - Can you provide details about the lock shape of capsules?
- The following illustrations outline the lock shape of hard capsules.
Shape of hard capsules
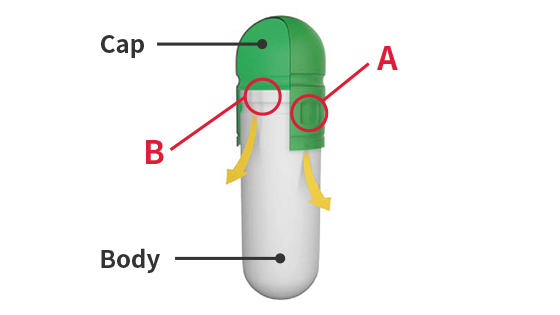 A:Caps are made with Qualicaps' proprietary pre-lock design to prevent capsules from breaking open during transportation.B:The groove on the body is tapered to minimize contact between the cap and body when filling, which prevents the capsule opening from cracking or peeling.
A:Caps are made with Qualicaps' proprietary pre-lock design to prevent capsules from breaking open during transportation.B:The groove on the body is tapered to minimize contact between the cap and body when filling, which prevents the capsule opening from cracking or peeling.Pre-lock (for delivery)
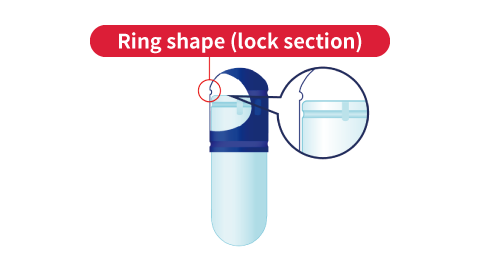
Final lock (after filling)
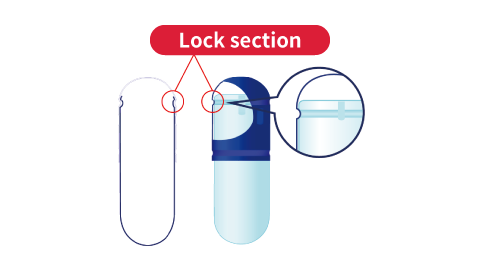 The cap and lock section (groove) of the body mesh together to lock the capsule.
The cap and lock section (groove) of the body mesh together to lock the capsule.
- What capsule sizes are available?
- The main sizes that Qualicaps® offers are as follows.
Pharmaceutical capsules are also available in size 9 for small animals.Pharmaceutical capsules:00, 0, 1, 2, 3, 4, 5Health food capsules:0, 1, 2, 3
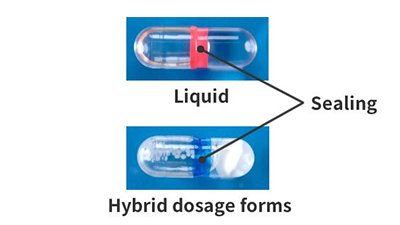
See the sizes on each product page for the capsule volume. - Can capsules be sealed?
- Yes, any type of capsule can be sealed. In addition to advantages like preventing capsules from being opened, the coloring of the seal also enhances distinguishability. Qualicaps® also sells fully-automatic capsule sealing machines.
- How should Qualicaps®' capsules be stored?
- The recommended storage conditions are as follows.
Recommended storage conditions
Gelatin capsules:Airtight with temperature 15 to 30 ℃,
humidity 40 to 60 % RHHPMC capsules:Airtight with temperature 15 to 30 ℃,
humidity 30 to 50 % RHPrecautions concerning handling-
Use pallets instead of placing on floor. -
Keep away from walls.
(around 10㎝) -
Avoid sudden temperature changes. -
Keep away from heat sources.
(around 1m)
-
- How long is the Qualicaps®' warranty period for capsules?
- The warranty is for three years after manufacture (outside of Japan), when capsules are kept unopened and stored at the recommended storage conditions after shipping.
Recommended storage conditions
Gelatin capsules:Airtight with temperature 15 to 30 ℃,
humidity 40 to 60 % RHHPMC capsules:Airtight with temperature 15 to 30 ℃,
humidity 30 to 50 % RHPrecautions concerning handling-

Use pallets instead of placing on floor. -

Keep away from walls.
(around 10㎝) -

Avoid sudden temperature changes. -

Keep away from heat sources.
(around 1m)
-
- Can you provide details about the elution characteristics of gelatin capsules?
- Gelatin capsules dissolve in water within ten minutes. See the test data on each product page for detailed data.
- Can you provide details about the elution characteristics of HPMC capsules?
- HPMC capsules dissolve in water within ten minutes. QUALI-V®-S acid-resistant capsules for health foods (HPMC capsules) do not dissolve readily in acidic solutions. This makes them suitable for ingredients for which the effectiveness is reduced by acidity. See the test data on each product page for detailed data.
- Is there a difference in brittleness between gelatin capsules and HPMC capsules?
- There is no difference in brittleness with either type when kept under the recommended storage conditions.
There is almost no change to HPMC capsules, even if the relative humidity is below these storage conditions. While gelatin capsules are more prone to breaking, QUALI-G™-PEG provides better resistance to brittleness, resulting in less broken capsules.
See the support data in the “Pharmaceutical Capsules Catalog” for more detailed data.Recommended storage conditions
Gelatin capsules:Airtight with temperature 15 to 30 ℃,
humidity 40 to 60 % RHHPMC capsules:Airtight with temperature 15 to 30 ℃,
humidity 30 to 50 % RHPrecautions concerning handling-

Use pallets instead of placing on floor. -

Keep away from walls.
(around 10㎝) -

Avoid sudden temperature changes. -

Keep away from heat sources.
(around 1m)
-
- What is hypromellose?
- "Hypromellose" is the official name in Japanese Pharmacopoeia, and refers to hydroxypropyl methylcellulose (HPMC). Hypromellose is made with pulp as raw material, and is widely used as pharmaceutical excipients and food additives. The QUALI-V® series are the world's first hypromellose-based capsules developed by Qualicaps®.
- Can you provide details about the materials used to make HPMC capsules?
- In addition to HPMC, the capsules are made using materials like carrageenan (gelling agent) and potassium chloride (gelling promoter).
Pharmaceutical Capsule FAQ
- Can you provide details about the space on capsules that can be printed?
- Refer to the image below. The space that can be printed varies with each product, so please send an inquiry using the "Contact" link at the top right of this page.
Offset printing

UV lazer printing

- Are your capsules listed in the Japanese Pharmacopoeia?
- QUALI-G™, QUALI-G™-PEG, QUALI-V®, and QUALI-V®-I are listed as “Capsules” and “Hypromellose Capsules” in the Japanese Pharmacopoeia.
- Do your pharmaceutical capsules comply with three prime regional Pharmacopoeia (the JP, the USP, and the Ph. Eur.)?
- Only the JP includes capsules, and Qualicaps®' pharmaceutical capsules comply with the JP.
We can provide products that comply with the JP, the USP/NF, and the Ph. Eur upon request. - What is the BSE/TSE risk assessment of pharmaceutical capsules made using bovine gelatin?
- The bovine gelatin used by Qualicaps® does not contain risk cow parts defined by the Standards for Raw Materials Originating from Living Organisms (Ministry of Health, Labour and Welfare Notification 210, May 20, 2003). The capsules are also manufactured using processes that reduce infectiveness, so there is unlikely to be any risk of BSE/TSE infection.
- Are your capsules registered in the Drug Master Files as pharmaceutical capsules?
- Qualicaps®' pharmaceutical capsules (QUALI-G™, QUALI-G™-PEG, QUALI-V®, QUALI-V®-I) are registered on Drug Master Files for the US and Canada. QUALI-V® and QUALI-V®-I are also registered on Center for Drug Evaluation in China.
- In what cases are capsules for inhalation used?
- QUALI-V®-I capsules for inhalation are mainly used for the treatment of respiratory diseases.
- Is there any powder residue with capsules for inhalation?
- The QUALI-V®-I capsules for inhalation are designed to have less powder left on the capsule wall.
See the support data in the “Pharmaceutical Capsules Catalog” for more detailed data.
Health Food Capsule FAQ
- Can you provide details on the materials used to make acid-resistant capsules for health foods (HPMC capsules) QUALI-V®-S?
- In addition to HPMC, the capsules are made using materials like gellan gum (gelling agent) and potassium chloride (gelling promoter).
- Do health food capsules contain any materials that may cause allergies?
- Gelatin capsules are free of allergens except gelatin (*28 raw materials identified as allergens in Japan). HPMC capsules are free of allergens.
- Do capsules contain any substances that are included in the Anti-Doping Code?
- These substances are not used as raw materials or during the manufacturing process, and as such are not included in the capsules.
- Do your capsules contain gluten?
- These substances are not used as raw materials or during the manufacturing process, and as such are not included in the capsules.
- Are capsules affected by radioactive contamination?
- Qualicaps® has verified that the source of all raw materials used in capsules and the location of manufacturers are outside the danger zones (Evacuation order zone, Planned evacuation zone) designated by the Japanese government.
Trademark
QUALI-V is a registered trademark of Qualicaps in China, India, US, Canada, EU, Switzerland, and UK.